InfoBatch®
Flexible Electronic Batch ReportingIntegrate
Acquire data from manufacturing systems, databases and historians at report run-time.
Aggregate
Contextualize information according to specified data models.
Report
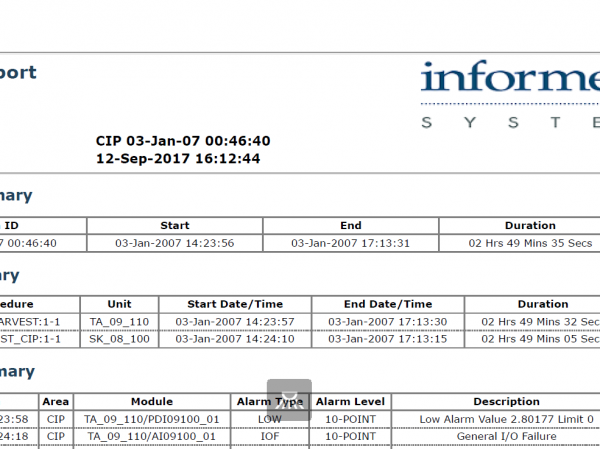
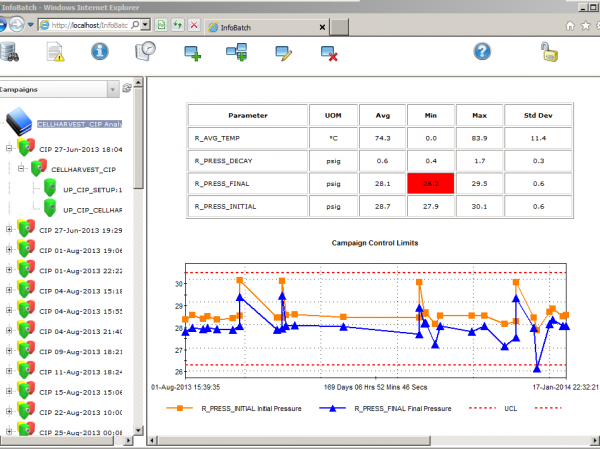
Generate comprehensive reports with flexible content and layout.
InfoBatch is a manufacturing reporting suite that simplifies compliance documentation, production reporting and process analytics across many industries. The world’s leading manufacturing facilities have improved productivity and reduced costs with InfoBatch. It has allowed them to:
Ensure Data Integrity
InfoBatch is designed for industries subject to Good Manufacturing Practice (GMP), ensuring compliance with regulations such as FDA 21 CFR Part 11, Electronic Records and Signatures, as well as data integrity guidance.
Enterprise Ready
InfoBatch is a robust server-based manufacturing reporting software suite. The software is scalable to ensure that a high volume of comprehensive reports can be reliably generated.
Easy Configuration
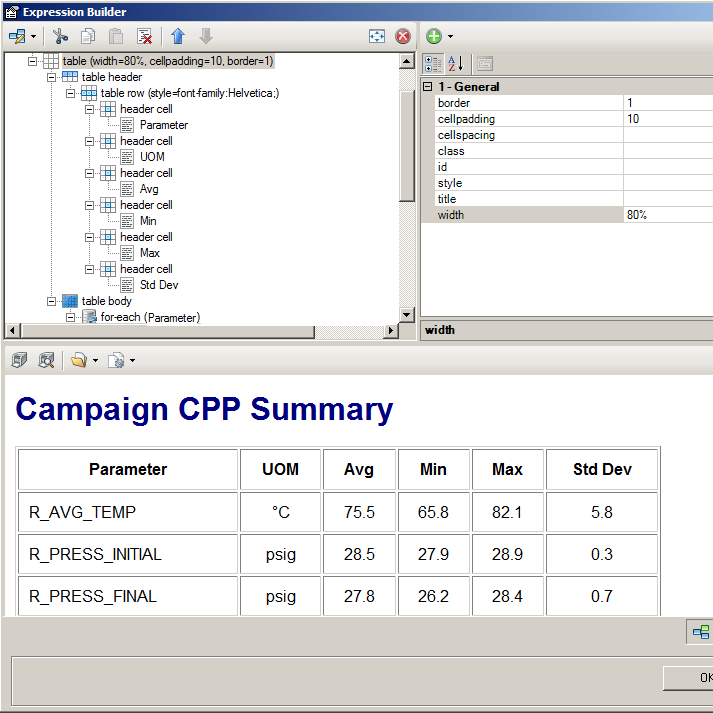
InfoBatch features a structured Configurator that enables authorized users to configure and maintain reports under change control. The Configurator features point-and-click data selection and an interactive report layout builder.
Out-of-the Box Support for Emerson DeltaV™ and Syncade™
InfoBatch provides pre-configured connectors for Emerson DeltaV and Syncade, enabling comprehensive batch reports to be deployed in less time and lower cost.