Electronic Batch Reporting
Electronic Batch Reporting
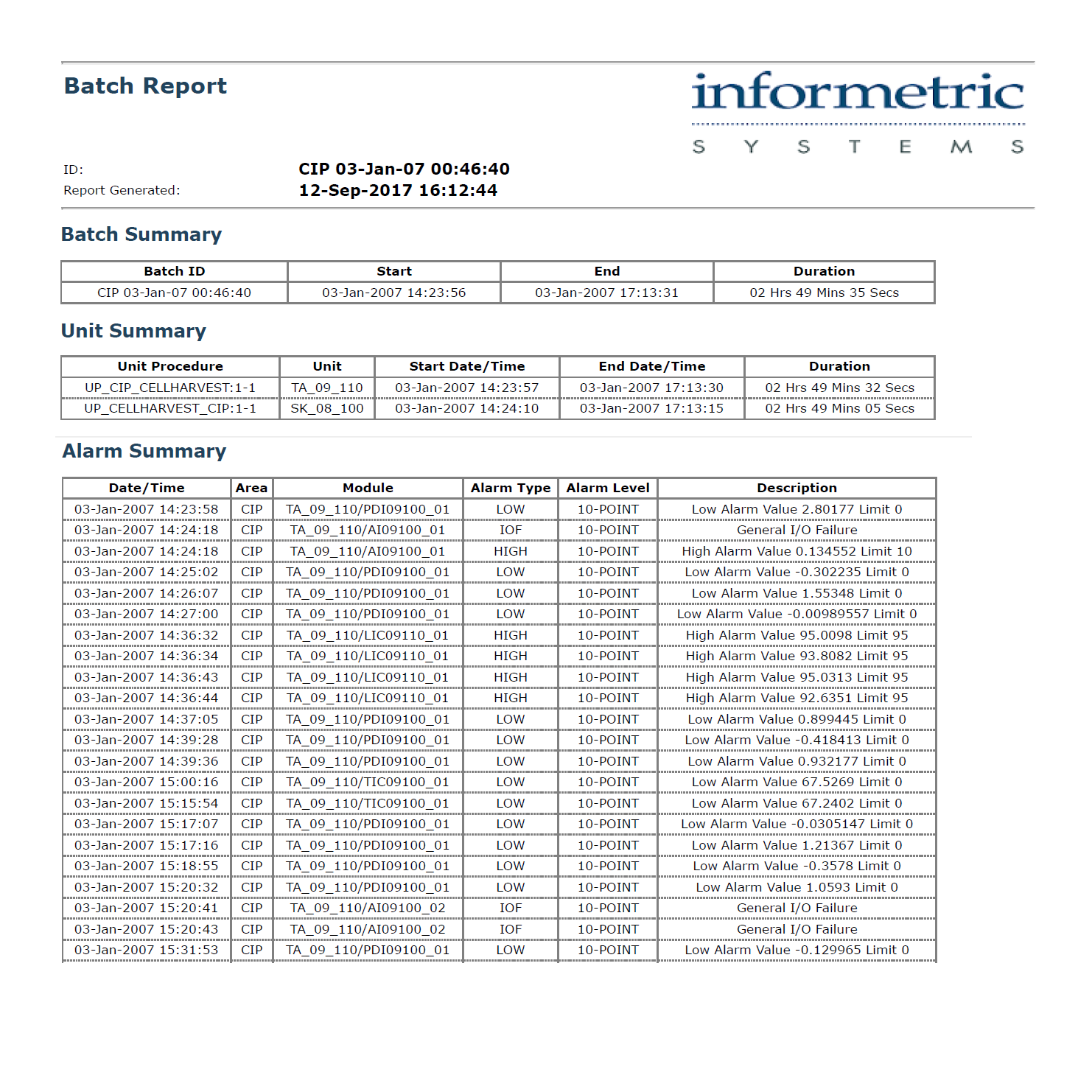
InfoBatch® Electronic Batch Reports streamline product release and compliance reporting. InfoBatch acquires manufacturing data from historians, laboratory systems and event databases in order to create a comprehensive batch record.
Reports can be as concise as an exception report or as comprehensive as a step-by-step production report. An intuitive report configurator combined with an advanced layout engine gives each manufacturer full control over the report content.
Reports can be generated on demand through a secure web application, or created automatically at the end of each batch using AutoGen™. InfoBatch can also be used for ad hoc analysis of batch production history over long periods of time.
InfoBatch security and traceability features make it suitable for regulatory reporting in facilities that must conform to Good Manufacturing Practices (GMP). The leading manufactures in life science industries including pharmaceuticals, biotech and medical devices rely on InfoBatch to ensure FDA compliance.
Request Demo