Manufacturers in regulated industries are facing increased focus on audit trail review. FDA guidance states that personnel responsible for record review should likewise review audit trail records, at a frequency consistent...
Read MoreNews and Events Page
Informetric is pleased to announce InfoBatch® 4.1. This latest release of InfoBatch incorporates features to increase report scalability and minimize report execution time. Revamped Document Control InfoBatch 4.1 incorporates a new server-based...
Read MoreDue to high demand, Informetric has added a second virtual InfoBatch™ Introductory Training course, the week of December 5th – 9th, 2022. Click here to learn more and register. ...
Read MoreInformetric will be at Emerson Exchange in Grapevine, TX next week! Visit us at booth 39 during the technology exhibits to learn about InfoLog™ or AgileDoc®, or to get a...
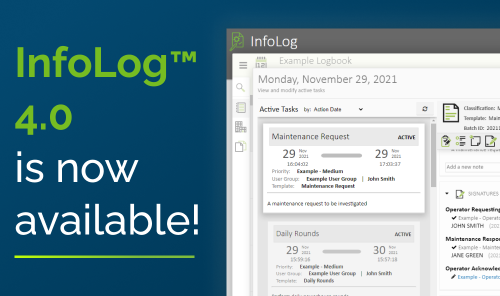
Read MoreInformetric Systems and Emerson Automation Solutions have expanded their partnership with the transfer of Emerson’s DeltaV™ Logbooks and Syncade™ Logbooks to Informetric. InfoLog® 4.0 is the result of that partnership....
Read MoreWe are excited to announce the release of the AgileDoc® Comparison Tool. AgileDoc is a Software Lifecycle (SLC) documentation suite. AgileDoc automatically generates documents from system source code, thus improving speed...
Read MoreInformetric Systems and Emerson are expanding their partnership with the transfer of Emerson’s DeltaV Logbooks and Syncade Logbooks to Informetric. Emerson and Informetric entered into an agreement in which Informetric...
Read More