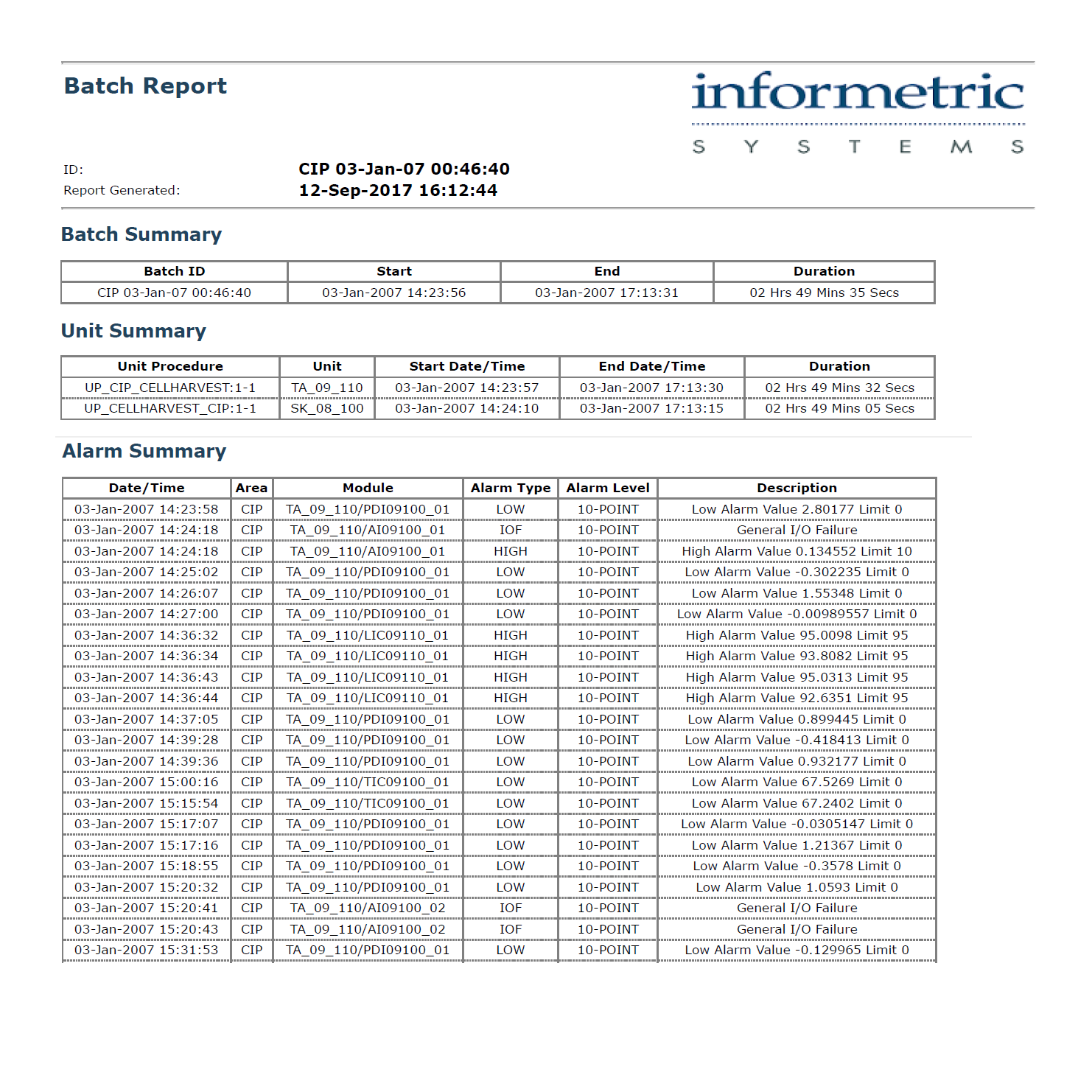
InfoBatch® Electronic Batch Reports streamline product release and compliance reporting. InfoBatch acquires manufacturing data from historians, laboratory systems and event databases in order to create a comprehensive batch record.
InfoBatch for Electronic Batch Reporting
InfoBatch® Electronic Batch Reports streamline product release and compliance reporting. InfoBatch acquires manufacturing data from historians, laboratory systems and event databases in order to create a comprehensive batch record. Reports can be as concise as an exception report or as comprehensive as a step-by-step production report. An intuitive report configurator combined with an advanced layout engine gives each manufacturer full control over the report content.
Reports can be generated on demand through a secure web application, or created automatically at the end of each batch using AutoGen™. InfoBatch can also be used for ad hoc analysis of batch production history over long periods of time.
InfoBatch security and traceability features make it suitable for regulatory reporting in facilities that must conform to Good Manufacturing Practices (GMP). The leading manufacturers in life science industries including pharmaceuticals, biotech and medical devices rely on InfoBatch to ensure FDA compliance.
Features
Compatibility
InfoBatch conforms to industry standard data access protocols which ensures compatibility with most process historians, LIMS, MES, and ERP systems. The software can easily be deployed on existing information systems, thus avoiding expensive and disruptive upgrades.
All-encompassing
InfoBatch reports can address virtually every manufacturing activity including batch release, APR investigation, morning production reports, cycle time analysis, validation studies and periodic assessment.
Simple to configure and use
User-friendly Windows® and web browser client applications reduce training costs and encourage full utilization.
Reports created from the source
InfoBatch does not replicate data. It retrieves data from the original source each time a report is generated, thus eliminating a point of failure associated with replication.
Configured, not programmed
All InfoBatch configuration tasks are simple point-and-click operations. Reports are generated with less training, lower development costs and fewer errors.
Regulatory Compliance
User security and traceability features are an integral part of the InfoBatch design, ensuring compliance with cGMP regulations and guidelines.
Resources
No matter what stage your data is in—paper, files, electronic logs, embedded databases, or automation systems—wrangling the wide variety of data sources that impact batch records is never easy. There can be so many systems capturing data, and they aren’t necessarily incorporated into a single repository. You need to connect those systems and contextualize that information in a meaningful way.
Life science manufacturers are mobilizing with unprecedented speed to release effective COVID-19 vaccines. The science behind the vaccines is impressive itself, but the speed and scale with which manufacturers are developing and producing billions of dosages is remarkable.

