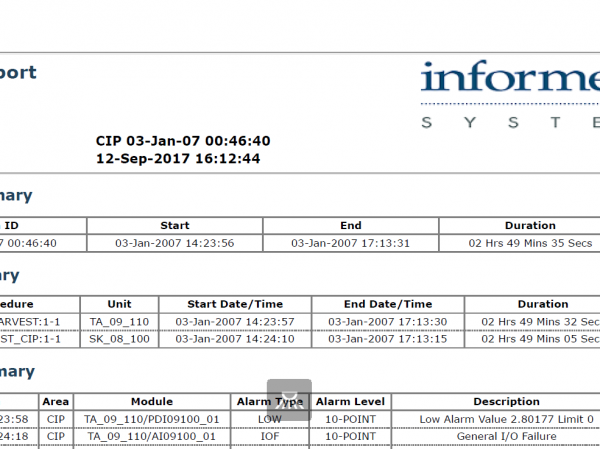
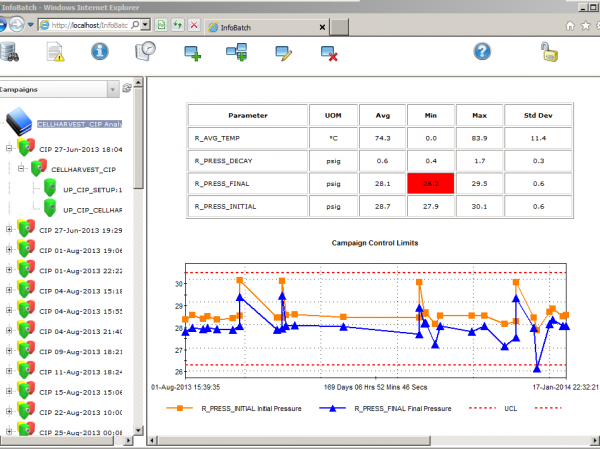
InfoBatch®
Flexible Electronic Reporting
InfoBatch is a manufacturing reporting suite that simplifies compliance documentation, production reporting and process analytics across many industries. The world’s leading manufacturing facilities have improved productivity and reduced costs with InfoBatch.

InfoBatch®
Flexible Electronic Reporting
InfoBatch is a manufacturing reporting suite that simplifies compliance documentation, production reporting and process analytics across many industries. The world’s leading manufacturing facilities have improved productivity and reduced costs with InfoBatch.
Serving Major Pharmaceutical Sites for Over 20 Years: 9 out of the 10 Largest Life Sciences Companies Use InfoBatch






Serving Major Pharmaceutical Sites for Over 20 Years: 9 out of the 10 Largest Life Sciences Companies Use InfoBatch




What sets InfoBatch apart:
9
of the top 10 life sciences companies
20
years of GMP industry experience
75%
reduction in validation cost effort
83-98%
faster report generation
What sets InfoBatch apart:
9
of the top 10 life sciences companies
20
years of GMP industry experience
75%
reduction in validation cost effort
83-98%
faster report generation
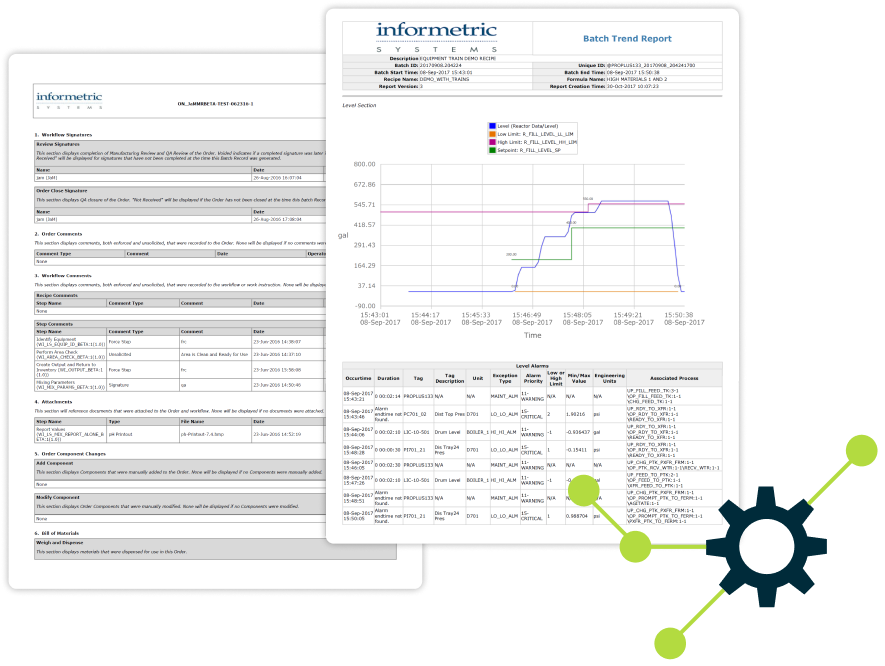
Purpose-Built for GMP Industries
InfoBatch has driven reporting for major sites in the pharmaceutical industry for over 20 years. A default, safe choice for batch reporting, the InfoBatch product undergoes continuous improvement while preserving backward compatibility for the facilities that have relied on it for decades.
Informetric’s extremely skilled support team understands the industry and are experts at keeping batch reports running 24/7.
Connect & Contextualize Data from Multiple Sources
InfoBatch uses context to aggregate data from multiple sources at report time, with no data replication. This significantly reduces the effort needed for data integration into a single reporting source.
InfoBatch’s data sources are integrated with the hierarchy in the data model, so no queries are issued to external connections.
Version-Controlled, Auditable Configuration
Become audit-ready with ease: InfoBatch offers version-controlled, auditable configuration – without files! With traceability on all aspects of configuration, compliance risk is eliminated. InfoBatch’s industry-specific features make lifecycle management straightforward for enterprise sites.
The InfoBatch Library Manager feature creates reusable report configurations, streamlining standardization. Its flexible, reusable report formatting can reduce validation cost effort by up to 75%.
Robust, Reliable, & Scalable Reporting Engine
InfoBatch reliably generates multi-thousand page reports containing rich content.

Faster Reports
Time is money, and facilities should not have to worry about their reports failing. InfoBatch is optimized for enterprise deployments in GMP environments: InfoBatch reports generate anywhere from 20 minutes to hours faster than other approaches.
Faster access to data and reports results in quicker decisions.
InfoBatch Content Resources

Many pharmaceutical manufacturers handle batch record production and review with tools not intended for that purpose, resulting in challenges aggregating the required batch record documentation and in working through the review and approval activity associated with batch release. Explore a few of the common problems manufacturers may face and how they can be solved with purpose-designed tools.

Life science manufacturers are mobilizing with unprecedented speed to release effective COVID-19 vaccines. The science behind the vaccines is impressive itself, but the speed and scale with which manufacturers are developing and producing billions of dosages is remarkable.

No matter what stage your data is in—paper, files, electronic logs, embedded databases, or automation systems—wrangling the wide variety of data sources that impact batch records is never easy. There can be so many systems capturing data, and they aren’t necessarily incorporated into a single repository. You need to connect those systems and contextualize that information in a meaningful way.
Out-of-the Box Support for Emerson DeltaV™ and Syncade™
InfoBatch provides pre-configured connectors for Emerson DeltaV and Syncade, enabling comprehensive batch reports to be deployed in less time and lower cost.