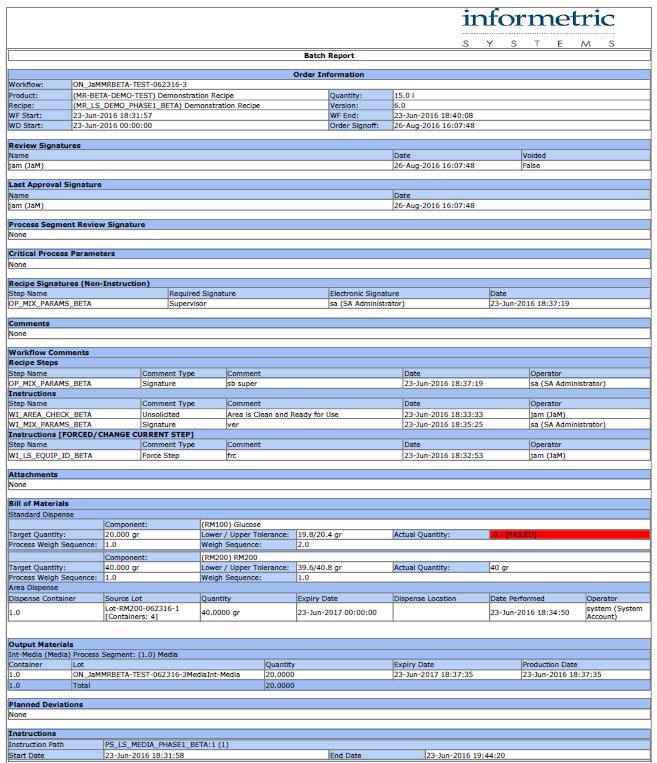
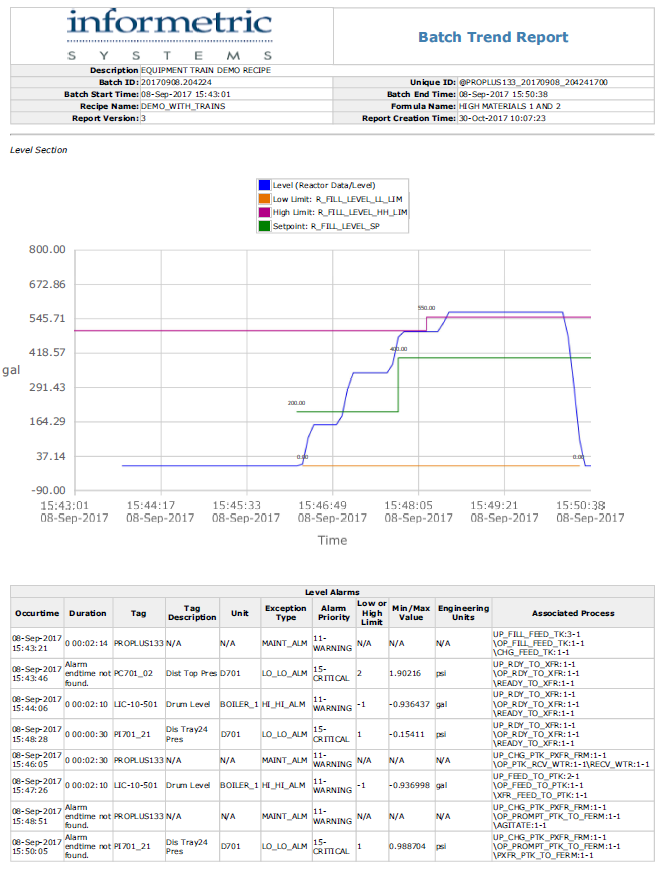
InfoBatch® for DeltaV and Syncade


Now Emerson’s Syncade manufacturing execution system is integrating InfoBatch to increase reporting efficiency at Level 3.
While cutting reporting time in half, InfoBatch also has built-in features that simplify FDA compliance and allow for an easier batch release process.